Full version 1 min
[ Diagnostic ]
Remote patient measurement ‘reliable’ for postvoid residual bladder volume
By Lucy Piper, medwireNews Reporter
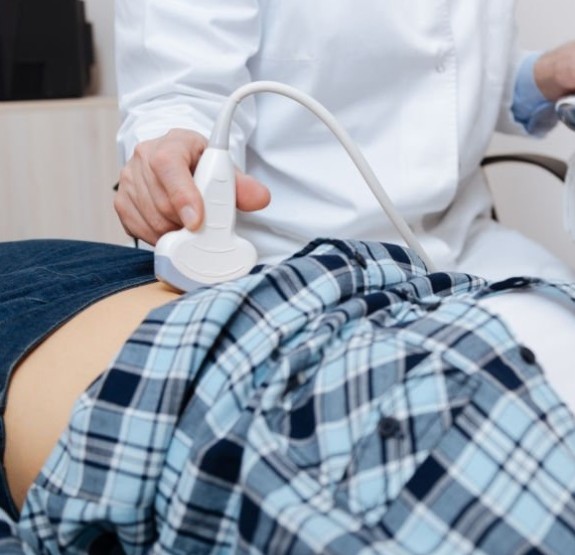
They also found that questionnaire responses indicated “a largely positive experience with learning and performing [bladder] PVR self-measurement,” with 74% of study participants preferring self-monitoring over measurement by their healthcare provider.
Study Design and Participant Characteristics
As reported in Urology in December 2023, 50 patients were enrolled during urology outpatient visits and randomly assigned to measure their own bladder PVR volume using the portable Butterfly iQ+ (Butterfly Network, Burlington, Massachusetts, USA) probe system first in US image mode and then in abstract image mode, or vice versa. A short tutorial on how to use the system was provided. The participants were aged a mean of 64 years, 80% were men, and 88% were White.
Reliability Assessment and Comparison with FDA-Approved Scans
The researchers report that patient measurement of bladder PVR volume using either US image or abstract image mode demonstrated “excellent reliability” compared with US FDA-approved bladder scans and healthcare provider measurement with the remote US probe. The intraclass correlations for patient measurements were 0.95–0.98, compared with 0.97–0.98 for provider measurements.
Comparing Patient and Provider Measurements for Bladder Residual Volume
Bladder PVR volume measurements by patients, compared with standard bladder scans, were a mean 7.50 mL higher using US image mode and 9.34 mL higher using the abstract image mode, which was within the predefined 50 mL threshold deemed clinically acceptable.
Compared with measurements made by providers using the remote probe, the differences were a mean 5.67 mL lower using the US image mode and 5.01 mL lower using the abstract image mode.
Assessment of Agreement and Limits of Agreement (LoA)
Cavallo et al point out that the range of difference for each of the comparisons exceeded the 50 mL threshold. For instance, the 95% limits of agreement (LoA) between standard bladder scanner and patient self-measurement were –71.73 mL and 86.73 mL using US image mode and –93.84 mL and 112.52 mL using abstract image mode.
However, they note that the LoA are “comparable to previous studies comparing ultrasound bladder scanners directly to voided urine volume.” In addition, “when PVR measurements from exiting FDA-cleared bladder scanners were compared to PVR measurements by ultrasound or by bladder catheterization in the hands of healthcare providers, similarly broad LoA were found,” they comment.
Comparison Across Demographic and Clinical Characteristics
Overall, there was no significant difference in mean PVR volume between any of the methods after stratifying patients by sex, age, BMI, and dexterity,
Patient Preferences and User Experience
In questionnaire responses, 58% of patients preferred the abstract image mode over the US image mode. And for both methods, at least 80% of patients felt the learning curve was “moderately easy” or “very easy” and at least 70% gave the same ratings for ease of use.
Conclusion and Implications for Practice
The study authors conclude that patient self-measurement using remote portable US devices “would enable longitudinal assessments of repeated PVR measurements in real-world settings, data-driven decision-making regarding need and timing of intervention, and provide patients with more opportunities to utilize telemedicine according to their needs and preferences.”
News stories are provided by medwireNews, which is an independent medical news service provided by Springer Healthcare Ltd. © 2024 Springer Healthcare Ltd, part of the Springer Nature Group
Read the article here: Urology 2023; doi:10.1016/j.urology.2023.11.026
HQ--02-24-2400013 02/2024. To access to the complete publication, please contact medical_information@pierre-fabre.com